The “Safe and Effective” Moderna Covid Vaccine for Infants Might be Neither
The more I look, the less I like their safety data.
I thought I was done writing about the Moderna and Pfizer vaccines for infants and toddlers with my prior piece on the subject. “Safe, not very effective, but let parents desperate to protect their kids have the choice,” would be the one line summary. However, one of my readers pointed out the rather awful rate of severe events in the Moderna 6 month to 2 year old group relative to placebo and I investigated. As a physician who prefers boosting vaccines over bashing them, I did not much care for what I found.
The 190 page document the FDA shared with the public from their review process of the Moderna Covid-19 vaccine for children 6 months to 18 years old covered a lot of ground. Skimming it through the first, and second, times, I didn’t see anything that looked like a poor safety signal for the youngest cohort. Yes, severe events were unbalanced amongst vaccinated infants, but there were three times as many as in the placebo cohort, and almost nothing looked like the sort of vaccine adverse reaction we might expect. But what were we expecting?
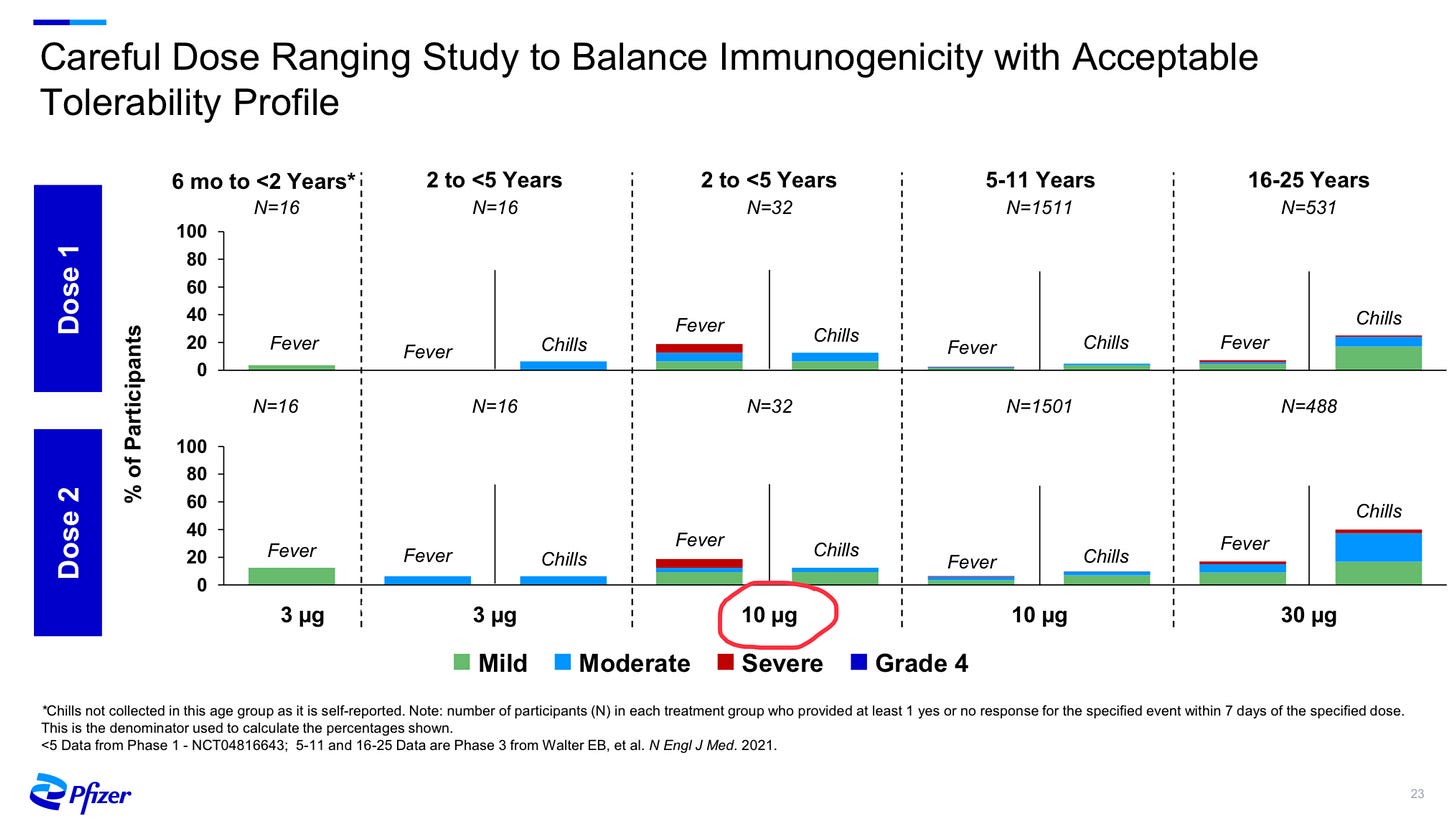
To be honest, our pre-trial expectation might have been: a lot of adverse reactions. Think again about how Pfizer felt compelled to drop their dosage in this age group to 3mcg, based on the rates of fever with a 10mcg dose in the 2-5 year olds:
We’ve been told consistently that Pfizer and Moderna are roughly equal microgram-to-microgram, so if Pfizer was uncomfortable rolling out a 10mcg dose in children under 5 years old, you can bet I would be nervous about adverse effects with a 25mcg Moderna dose in 6 month olds!
What the FDA documents reveal, however, is an acceptable safety profile — at first glance. I mean, we expect a higher rate of adverse events in a group given an active drug compared to placebo; that’s normal. The only concern is if the adverse event is rare but extremely dangerous (i.e., the potentially fatal blood clots from the adenovirus-vector vaccines mostly in younger women) or common enough to possibly outweigh the good they accomplish in disease prevention (i.e., post-mRNA second shot myocarditis in young, healthy men). Honestly, it’s hard to know where to stand with the Moderna 25mcg dose for infants.
On the one hand, there was one “run-of-the-mill” adverse event, attributed both by study investigators and the FDA to the vaccine, in this group: a case of febrile seizure on the day after vaccination. (Febrile seizures are terrifying for parents, sometimes lead to a brief hospitalization, but rarely yield long-term consequences.) Another infant with a family history of diabetes experienced the onset of type 1 diabetes, rather surprisingly linked to the vaccine by study investigators, but not by the FDA. Google “can vaccines cause type 1 diabetes?” if you need convincing this is a hot area of controversy in vaccinology. I’ll just say it might be possible, but it’s probably more possible that the child was destined to develop diabetes with one immune trigger or another eventually.
One or two infants out of 1800 experiencing a serious medical problem attributable to vaccination could be acceptable. After all, Covid-19 has put about 1 in 450 children under 5 in the hospital since the pandemic began (although the COVID-NET figures include the perhaps 15-30% of hospitalizations “with” Covid rather than “for” Covid, but I allow that distinction is anything but simple):
If these events were the only safety concerns for the 25mcg Moderna dose in this age cohort, parents and physicians would have a nuanced decision to make. Since the minority of American infants with health problems constitute roughly half of hospital admissions, a healthy infant would expect a risk well under 1 in 450, probably 1 in a few thousand. We also don’t know to what degree the Moderna vaccine will prevent hospitalization for SARS-CoV-2 infections; the study had no severe Covid-19 cases in either the vaccine or placebo groups. Certainly, it will be less than 100% effective, most of all in the children with compromised immune systems we most need to help; and whatever initial effect it has will likely wane over time. After all, to keep the comparison fair, the past 6 months were the worst of the pandemic for infant hospitalizations, but the cumulative Covid hospitalization rate over that time period was more like 1 in 1700; this would be the better comparator if the vaccine only protects for 6 months, and is remarkably similar to the 1 in 1800 rate of hospitalization for clear vaccine complications in the trial.
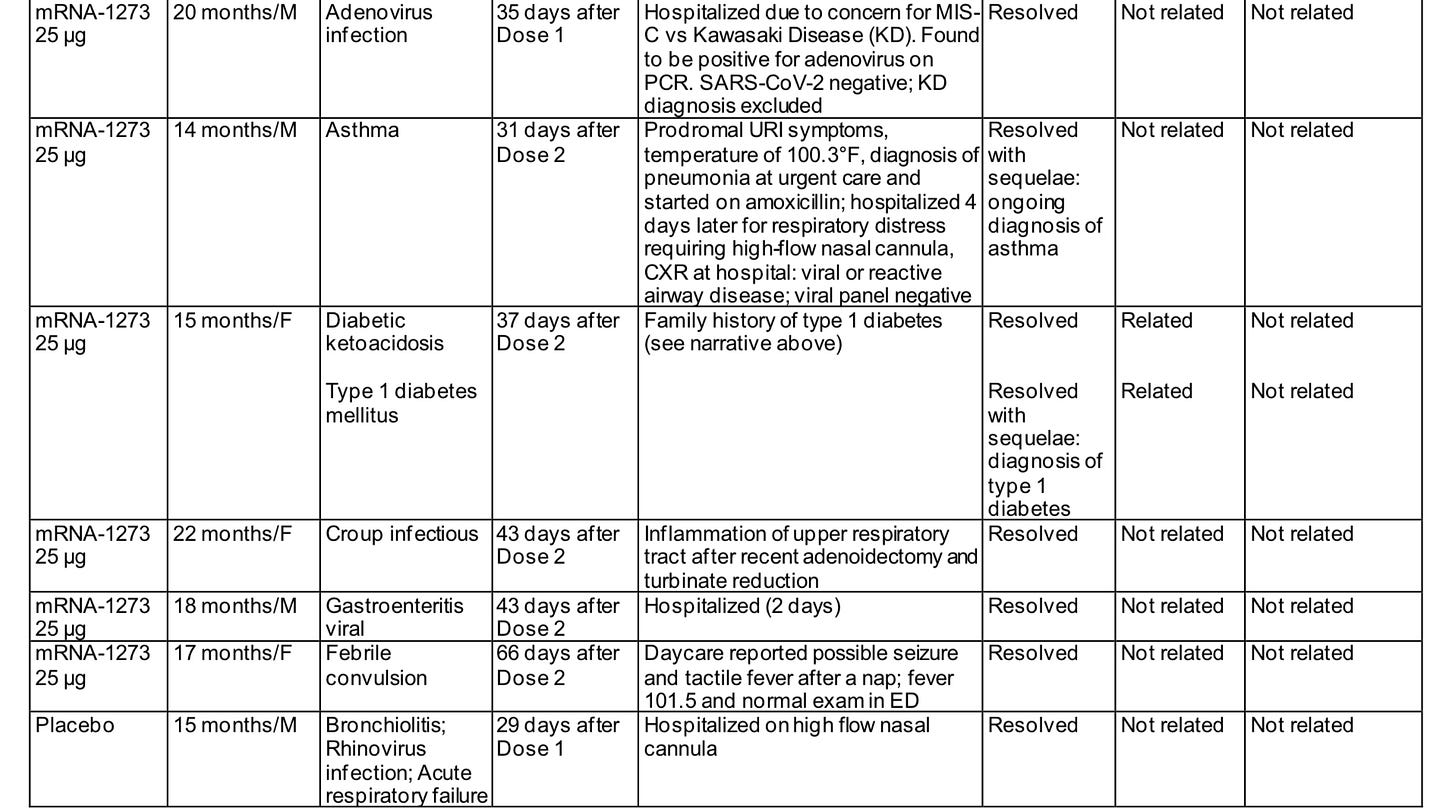
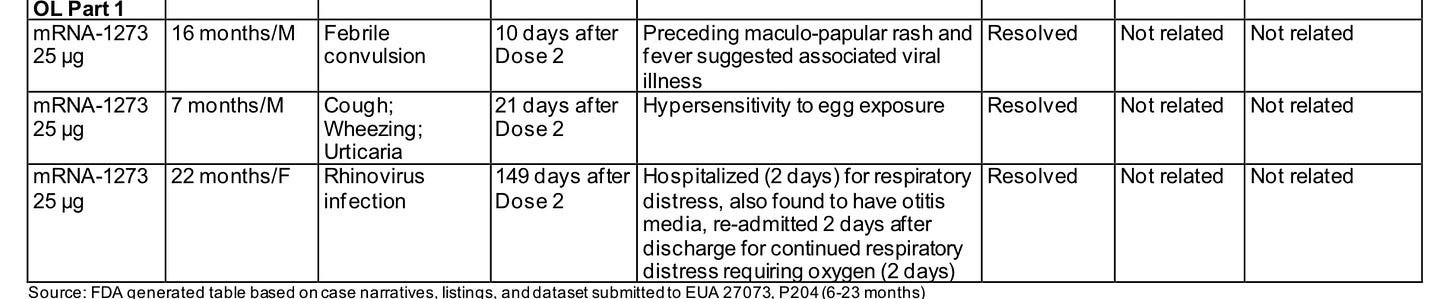
However, concerns for the Moderna dose really don’t end there. The all-cause hospitalization rate was profoundly higher in the vaccine group. It’s worth a glance:
The final tally, kicking out the foreign body which we are not going to try to blame on the vaccine, is 17 severe adverse events after vaccine (nearly 1 in 100 Moderna trial participants) vs 1 hospitalization for viral infection of the 600 placebo recipients. No one was hospitalized for Covid. Of those 17, we had 4 febrile seizures, 9 hospitalizations for infectious diseases, 3 serious autoimmune reactions (the erythema multiforme skin reaction, development of a hypersensitivity reaction to eggs, and the type 1 diabetes onset), and 1 serious case of croup.
Only the febrile seizure soon after vaccination was deemed related to the vaccine by the FDA. I don’t fault them for this adjudication. I would not expect this potpourri of adverse reactions many days or even weeks after vaccination to be vaccine induced, either. Possible explanations would include an imbalance in the time that the placebo group was followed versus the vaccine cohort, although that is not mentioned anywhere; also possible would be flaws in data collection with the placebo cohort (although a pharmaceutical company failing to collect data on adverse effects in the placebo arm of their trial would be strange indeed!). The most likely explanation would be coincidence, the perils of an undersized trial. We also have to allow some possibility, however, that an infant’s immune system might be unfavorably altered by a high dose of a novel vaccine, and it might affect their ability to avoid severe infection or make autoimmune reactions more likely for some time.
One of the great benefits of randomized controlled trials is that adverse events should balance out, and not be confounded by the real world issues we are now left to sort through. If hospitalizations are higher in infants who receive the Moderna vaccine than a matched cohort who do not, we can’t know whether that’s simply due to less healthy infants (rightly) being given this vaccine at a higher rate than healthier infants. We might never know the answer to this rather pressing question.
In a more perfect world, a physician like me would not have to rely on a Substack reader under a nom de plume like “Jack McJackers” to even identify a serious safety concern like this. This sort of potential safety signal should be out in the world, being discussed. Curious, I sifted through the 7.5 hour video of the FDA’s advisory committee meeting to see if the subject came up; at the 2 hour mark, Dr Ofer Levy shares these comments about the imbalance of serious RSV, croup and pneumonia cases in the Moderna group:
“I had a question about what appeared to be an imbalance with respect to RSV infections and pneumonia. And my question to you is, I mean, you know, a priori we might not think that that’s possible but on the other hand vaccines can have off-target effects, effects on the immune memory, but who knows - just looking at the data, is that a statistically significant higher RSV and pneumonia signal in the vaccine group?”
So, the concern was raised. It was parried by other VRBAC speakers noting that the overall rate of viral upper respiratory infections was actually higher in the placebo group since they had a higher rate of Covid-19 infection, 12.2% to 10.3%; and that the high number of reviewed events makes statistical analysis challenging (I think this is a very fair point, especially since we have two age subsets for two vaccine candidates to pick through for problems). Another unnamed speaker stated, “We did not see increased severity of these types of infection in the vaccinated group compared to the placebo group.” I am truly unsure what this person did “see.” Clearly, the overall rate of severe infections was higher in the vaccine group; the only question is whether it was simply failure of the trial to give us representative data.
One thing I can say with certainty: anyone claiming that the benefits of the Moderna 25mcg vaccine for this age group clearly outweigh its risks is not speaking the truth. I really don’t know what to say when someone who knows much more about the subject than I do, White House COVID-19 Response Coordinator Dr. Ashish Jha, makes a statement like this:
Seriously?
Again, I could handle the uncertainty for a frankly borderline safety concern if we knew this vaccine would make a substantial impact on Covid-19 disease burden, like the mRNA vaccines accomplished in the at-risk adult population, where they truly have been life-saving. However, we lack such confidence in the infant cohort.
Efficacy? Middling at best, even in a very short window after the second shot.
How about stopping transmission via reducing asymptomatic infections? Nope.
Helpful in the one high risk group for which the study gathered data, obese infants? Negative efficacy.
And, of course, while we expect at least moderate reduction in Covid-19 hospitalizations, the study was too small and brief to find any severe infections in either placebo or vaccine group. So, no data there.
Truly, it’s hard to make the case that this vaccine for this cohort makes sense even for a higher risk infant until/unless we had more safety and efficacy data. For a healthy infant, or the majority of infants in the U.S. who have already had Covid? Given annual hospitalization rates probably in the 1 in many 1000s for this group, it seems very unlikely that benefits outweigh risks for this particular vaccine.
I know the FDA, CDC and the White House have enthusiastically endorsed the Moderna Covid-19 vaccine for all infants. I think a system that is capable of such an endorsement is broken, and in dire need of fixing.








I think this might just be your "red pill" moment. I really enjoyed reading your process of re-evaluating the data and updating your opinion
“I know the FDA, CDC and the White House have enthusiastically endorsed the Moderna Covid-19 vaccine for all infants. I think a system that is capable of such an endorsement is broken, and in dire need of fixing.”
Really well put.
As a lay reader I don’t know what to conclude -- other than these trials are tiny and should’ve been bigger -- but it’s easy enough to see that the confident pronouncements from folks like Walensky and Jha bear little resemblance to the actual clinical trial submissions...
Where I live (California) there’s a proposed bill to punish doctors for spreading Covid vaccine misinformation. Presumably, adopting the position you’ve sketched in this article could be enough to get California doctors delicensed! Scary stuff.
Here’s to hoping cooler heads will prevail, doctors can advise as they see fit, and we stop rewarding cheerleading and re-focus on evidence as the basis of pharmaceutical regulation.