The "Paxlovid Rebound" Problem is Real
Not a "Mystery" or a "Puzzle" and we should have been warned about it.
I had low expectations for Pfizer’s anti-Covid medication, Paxlovid, before its clinical trial data was released. After all, novel antivirals face tough sledding, with only a handful of success stories over decades of research. But then — that data! An 80-90% reduction in severe disease among high risk patients given the drug early in their Covid-19 course was a remarkable result. Sure, a few warts have popped up since: the list of problematic drug-drug interactions has grown (including some heavily prescribed stalwarts among the high risk, like statins and blood thinners), dosing has been adjusted for those with kidney disease, and the preliminary numbers for a second trial in lower risk patients have been a bit less stellar, in the 70% ballpark. Still, though: an oral drug capable of massively reducing hospitalization and death in high risk Covid-19 patients? The only problem was the lack of availability!
There’s more to the story now, though. A host of anecdotal reports began popping up in the past week of people taking Paxlovid experiencing viral remission, negative rapid test and all, and then getting sick again - positive rapid test and all.
Through the magic of Twitter, the truth actually became obvious in real time — no puzzle-solving needed. The FDA was well aware of this rebound in viral loads in a substantial proportion of people treated with Paxlovid, around days 10-14 after starting treatment. For some reason, though, they didn’t think to tell us doctors about it. It’s not in the Fact Sheet for Health Care Providers, and there is only one line even vaguely referencing this possibility in the Fact Sheet for Patients:
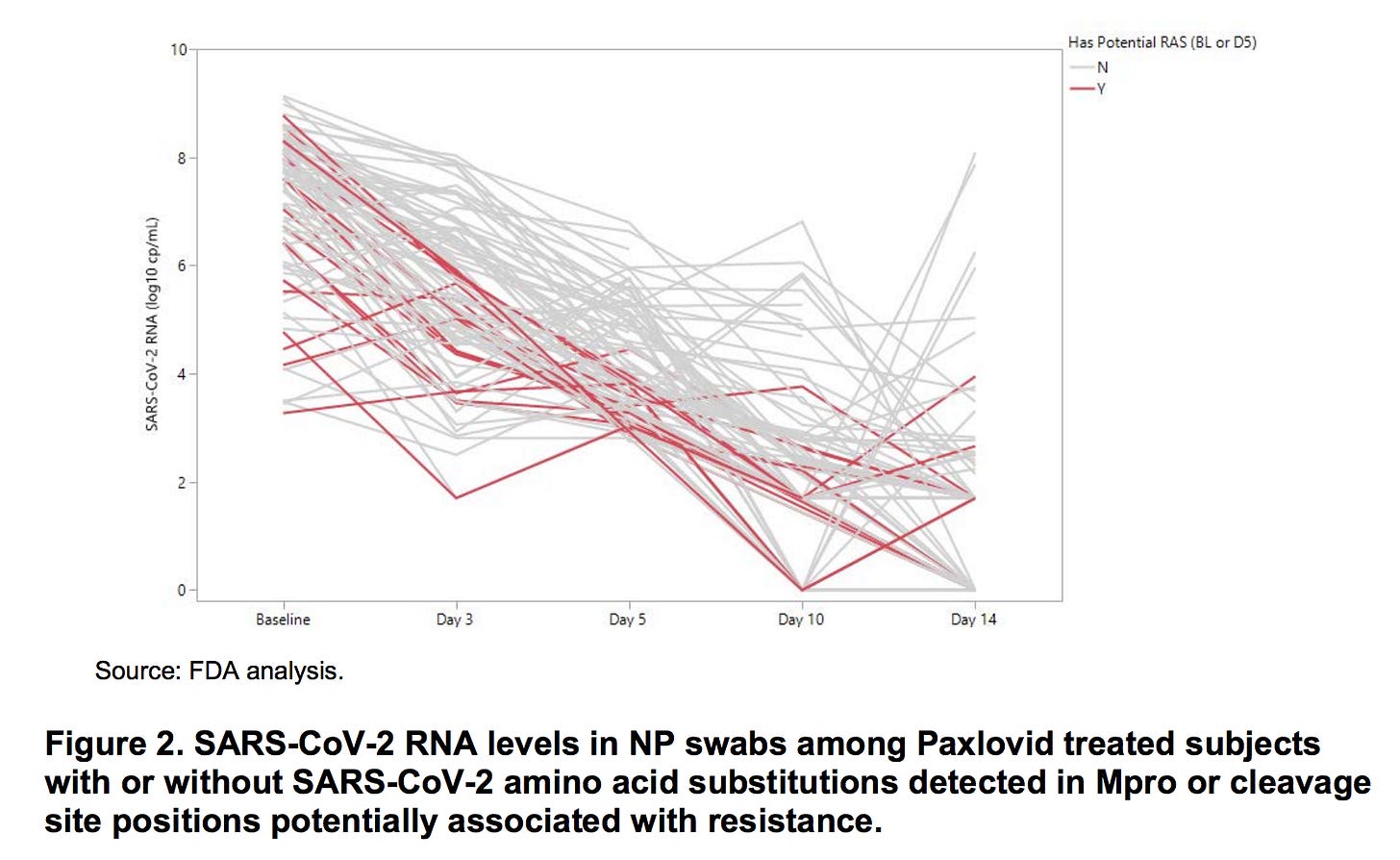
What did Pfizer and the FDA already know about Paxlovid rebound? On page 23 of their 120 page EUA Review, they shared the graph below, presumably (based on the labeling to the right of the graph) derived from the swabs of the 97 patients who received Paxlovid and were tested for mutations in the areas most likely to lead to resistance to Paxlovid’s replication-inhibiting activity. In red, are those with mutations; gray, those without. Most were tested for viral loads at days 1, 3, 5, 10, and 14.
The majority of patients had their viral load trend down nicely. Red lines roughly mirror gray lines; the point of the graph was, I think, to reassure us that the presence of mutations was not linked to the likelihood of rebound. However, I’m not reassured that among patients with swabs on day 10, 4 clearly have experienced rebounds with at least a 100-fold increase in viral load after their initial reduction. At day 14, another 7 have had a similar rebound. That’s 11 out of 97 patients - 11.3% - so not exactly an “outlier” result.
As to the question, “how did this not make it onto Paxlovid’s labeling information?”—I have no answer. At least, no answers that are not extremely cynical.
We absolutely should have been warning patients (and doctors) that rapid improvement, followed by clinical deterioration and, presumably, high risk of contagion, could happen in some 10% of high-risk patients. It’s also quite clear that we need more information about Paxlovid now.
For one: presumably Pfizer and the FDA know what happened to the trial subjects who saw their viral load rebound. Was this a mere annoyance, or did it increase the risk of hospitalization? If most or all of the small group of hospitalized patients who received Paxlovid (i.e., treatment failures) were in this rebound sub-group, it is highly possible that treating them with an additional course might have made the effectiveness of Paxlovid even better. In the FDA’s most recent approval letter to Pfizer, none of this information was requested or even mentioned; most of their emphasis was data on possible SARS-CoV-2 mutations from people treated with Paxlovid.
Secondly: many commentators have reminded us that the data we have on Paxlovid is pre-Omicron, and excluded vaccinated patients to allow for a smaller study. Pfizer tells us that Paxlovid works fine with Omicron, but this is based on unpublished, in vitro studies, not clinical trials. As to its effectiveness among vaccinated people, one would assume it would take a much larger study to ascertain this, since vaccinated people are much less likely to be severely ill in the first place. However, it appears Pfizer does not wish to share their data from the second trial on this rather enormous, important population, which is a loss for all of us wondering when to prescribe this drug:
Finally: Pfizer and the FDA need to update their messaging to include the obvious reality that people who take Paxlovid, and then see a recurrence of their symptoms, should assume themselves to be contagious, and seek medical counsel in the meanwhile. In the Boston Globe article, Dr Michael Charness shares his thoughts on the subject:
I tend to agree that another course of treatment, possibly even for longer than five days, would be prudent in these situations. Clearly, the FDA should ask Pfizer to include this intervention in their ongoing trials.
To be clear, this whole kerfuffle has me more disappointed in the FDA than in Paxlovid. The vast majority of patients experienced the expected, sustained drop in viral load that we would anticipate from an effective antiviral. The fact that outcomes were tracked for 28 days after the start of treatment, and we still saw almost none (0.7%) of of the treated, high-risk patients be hospitalized (vs 6.5% receiving placebo), implies that this rebound phenomenon might be immunologically concerning, and a problem in terms of counseling patients around contagiousness, but not a deal-breaker. In the clinical trials, Paxlovid succeeded at its most important task: preventing hospitalization and death.
I think the appropriate response at this point would be a mea culpa from the FDA — I don’t expect mea culpas from pharmaceutical giants — and a promise of better analysis of the data they already have, and request for further study from Pfizer in ongoing trials. I also think that, while these questions are being settled, Paxlovid is best reserved for people truly at high risk of severe disease, and not healthy people hoping for a Get Out of Jail Free card from an unpleasant bout of Covid.









Why take a brand new drug? Why not just take safe, cheap, and effective Ivermectin? I don't get it.
Great article and the topic is amazing!
Check out other paxlovid articles:
On April 13, I brought up many people having rebounds with Paxlovid
https://igorchudov.substack.com/p/paxlovid-snake-oil-of-the-21st-century
Brian Mowrey wrote several great paxlovid articles giving some biomolecular details
https://unglossed.substack.com/
Paxlovid was never tested on the vaxxed, ON PURPOSE, and no one should be surprised that it does not work on the vaxed.
Ivermectin, as always, works great and costs 1/100 of Paxlovid cost. I treated my wife with it to amazing results, temp dropped to normal right away and no long covid afterwards